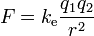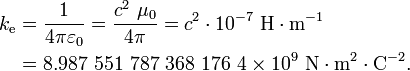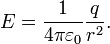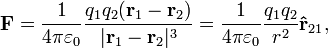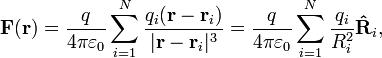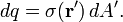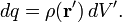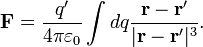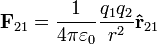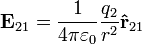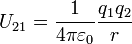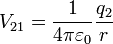Coulomb's law
From Wikipedia, the free encyclopedia
| Electromagnetism | |||||||
 |
|||||||
Electricity · Magnetism
|
|||||||
Coulomb's law is a law of physics describing the electrostatic interaction between electrically charged particles. It was studied and first published in 1783 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism. Nevertheless, the dependence of the electric force with distance (inverse square law) had been proposed previously by Joseph Priestley[1] and the dependence with both distance and charge had been discovered, but not published, by Henry Cavendish, prior to Coulomb's works.
Coulomb's law may be stated in scalar form as follows:
- The magnitude of the electrostatic force between two point electric charges is directly proportional to the product of the magnitudes of each of the charges and inversely proportional to the square of the distance between the two charges.
Contents[hide] |
[edit] Scalar form

The scalar form of Coulomb's law will only describe the magnitude of the electrostatic force between two electric charges. If direction is required, then the vector form is required as well. The magnitude of the electrostatic force (F) on a charge (q1) due to the presence of a second charge (q2), is given by
where r is the distance between the two charges and ke a proportionality constant. A positive force implies a repulsive interaction, while a negative force implies an attractive interaction.[2]
The proportionality constant ke, called the Coulomb constant (sometimes called the Coulomb force constant), is related to defined properties of space and can be calculated exactly:[3]
By definition in SI units, the speed of light in vacuum, denoted c,[4] is 299,792,458 m·s−1,[5] and the magnetic constant (μ0), is defined as 4π × 10−7 H·m−1,[6] leading to the consequential defined value for the electric constant (ε0) as ε0 = 1/(μ0c2) ≈ 8.854187817×10−12 F·m−1.[7] In cgs units, the unit charge, esu of charge or statcoulomb, is defined so that this Coulomb constant is 1 and dimensionless.
This formula says that the magnitude of the force is directly proportional to the magnitude of the charges of each object and inversely proportional to the square of the distance between them. The exponent in Coulomb's Law has been found to be equal to −2 with precision of at least 2.7±3.1×10−16.[8]
Coulomb's law can also be interpreted in terms of atomic units with the force expressed in Hartrees per Bohr radius, the charge in terms of the elementary charge, and the distances in terms of the Bohr radius.
[edit] Electric field
It follows from the Lorentz Force Law that the magnitude of the electric field (E) created by a single point charge (q) at a certain distance (r) is given by:
For a positive charge, the direction of the electric field points along lines directed radially away from the location of the point charge, while the direction is the opposite for a negative charge. The SI units of electric field are volts per meter or newtons per coulomb.
[edit] Vector form
In order to obtain both the magnitude and direction of the force on a charge, q1 at position 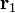 , experiencing a field due to the presence of another charge, q2 at position
, experiencing a field due to the presence of another charge, q2 at position 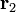 , the full vector form of Coulomb's law is required.
, the full vector form of Coulomb's law is required.
where r is the separation of the two charges. This is simply the scalar definition of Coulomb's law with the direction given by the unit vector,  , parallel with the line from charge q2 to charge q1.[9]
, parallel with the line from charge q2 to charge q1.[9]
If both charges have the same sign (like charges) then the product q1q2 is positive and the direction of the force on q1 is given by  ; the charges repel each other. If the charges have opposite signs then the product q1q2 is negative and the direction of the force on q1 is given by
; the charges repel each other. If the charges have opposite signs then the product q1q2 is negative and the direction of the force on q1 is given by 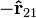 ; the charges attract each other.
; the charges attract each other.
[edit] System of discrete charges
The principle of linear superposition may be used to calculate the force on a small test charge, q, due to a system of N discrete charges:
where qi and 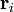 are the magnitude and position respectively of the ith charge,
are the magnitude and position respectively of the ith charge, 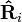 is a unit vector in the direction of
is a unit vector in the direction of 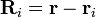 (a vector pointing from charge qi to charge q), and Ri is the magnitude of
(a vector pointing from charge qi to charge q), and Ri is the magnitude of 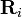 (the separation between charges qi and q).[9]
(the separation between charges qi and q).[9]
[edit] Continuous charge distribution
For a charge distribution an integral over the region containing the charge is equivalent to an infinite summation, treating each infinitesimal element of space as a point charge dq.
For a linear charge distribution (a good approximation for charge in a wire) where 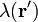 gives the charge per unit length at position
gives the charge per unit length at position 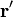 , and
, and 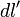 is an infinitesimal element of length,
is an infinitesimal element of length,
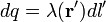 .[10]
.[10]
For a surface charge distribution (a good approximation for charge on a plate in a parallel plate capacitor) where 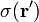 gives the charge per unit area at position
gives the charge per unit area at position 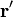 , and
, and 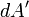 is an infinitesimal element of area,
is an infinitesimal element of area,
For a volume charge distribution (such as charge within a bulk metal) where 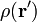 gives the charge per unit volume at position
gives the charge per unit volume at position 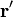 , and
, and 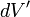 is an infinitesimal element of volume,
is an infinitesimal element of volume,
The force on a small test charge 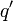 at position
at position 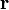 is given by
is given by
[edit] Graphical representation
Below is a graphical representation of Coulomb's law, when q1q2 > 0. The vector 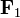 is the force experienced by q1. The vector
is the force experienced by q1. The vector 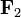 is the force experienced by q2. Their magnitudes will always be equal. The vector
is the force experienced by q2. Their magnitudes will always be equal. The vector 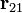 is the displacement vector between two charges (q1 and q2).
is the displacement vector between two charges (q1 and q2).
[edit] Electrostatic approximation
In either formulation, Coulomb’s law is fully accurate only when the objects are stationary, and remains approximately correct only for slow movement. These conditions are collectively known as the electrostatic approximation. When movement takes place, magnetic fields are produced which alter the force on the two objects. The magnetic interaction between moving charges may be thought of as a manifestation of the force from the electrostatic field but with Einstein’s theory of relativity taken into consideration.
[edit] Table of derived quantities
| Particle property | Relationship | Field property | |||||
| Vector quantity |
|
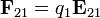 |
|
||||
| Relationship | 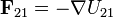 |
 |
|||||
| Scalar quantity |
|
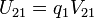 |
|
[edit] See also
- Biot-Savart Law
- Method of image charges
- Electric field
- Electric constant
- Coulomb, the SI unit of electric charge named after Charles Augustin de Coulomb
- Electromagnetic force
- Molecular modelling
[edit] Notes
- ^ Robert S. Elliott (1999), Electromagnetics: History, Theory, and Applications, ISBN 978-0-7803-5384-8, http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0780353846.html
- ^ Coulomb's law, Hyperphysics
- ^ Coulomb's constant, Hyperphysics
- ^ Current practice is to use c0 to denote the speed of light in vacuum according to ISO 31. In the original Recommendation of 1983, the symbol c was used for this purpose and continues to be commonly used. See NIST Special Publication 330, Appendix 2, p. 45
- ^ [1]
- ^ [2]
- ^ http://physics.nist.gov/cgi-bin/cuu/Value?ep0
- ^ Williams, Faller, Hill (1971), "New Experimental Test of Coulomb's Law: A Laboratory Upper Limit on the Photon Rest Mass", Physical Review Letters 26: 721–724, doi:10.1103/PhysRevLett.26.721, http://prola.aps.org/abstract/PRL/v26/i12/p721_1
- ^ a b c Coulomb's law, University of Texas
- ^ Charged rods, PhysicsLab.org
[edit] References
- Griffiths, David J. (1998). Introduction to Electrodynamics (3rd ed.). Prentice Hall. ISBN 0-13-805326-X.
- Tipler, Paul (2004). Physics for Scientists and Engineers: Electricity, Magnetism, Light, and Elementary Modern Physics (5th ed.). W. H. Freeman. ISBN 0-7167-0810-8.
[edit] External links
- Coulomb's Law on Project PHYSNET.
- Electricity and the Atom — a chapter from an online textbook
- A maze game for teaching Coulomb's Law—a game created by the Molecular Workbench software
- The inverse cube law The inverse cube law for dipoles (PDF file) by Eng. Xavier Borg
- Electric Charges, Polarization, Electric Force, Coulomb's Law Walter Lewin, 8.02 Electricity and Magnetism, Spring 2002: Lecture 1 (video). MIT OpenCourseWare. License: Creative Commons Attribution-Noncommercial-Share Alike.